Scientists have always been fascinated by the beauty and strength of diamonds. These precious stones have been coveted for centuries for their brilliance and durability. But what if we told you that scientists have now created a diamond that is even harder than natural diamonds? Yes, you read that right. In a groundbreaking development, researchers have successfully created a synthetic diamond with a hexagonal lattice, making it stronger and more durable than any natural diamond.
This revolutionary achievement was made possible by a team of scientists from the University of Bayreuth in Germany. Using a process involving high-pressure heating of graphene, they were able to produce a diamond that can withstand extreme pressures of up to 155 GPa and temperatures of 1,100 degrees Celsius. This is far beyond the durability of natural diamonds, which can only withstand pressures of up to 100 GPa and temperatures of 1,000 degrees Celsius.
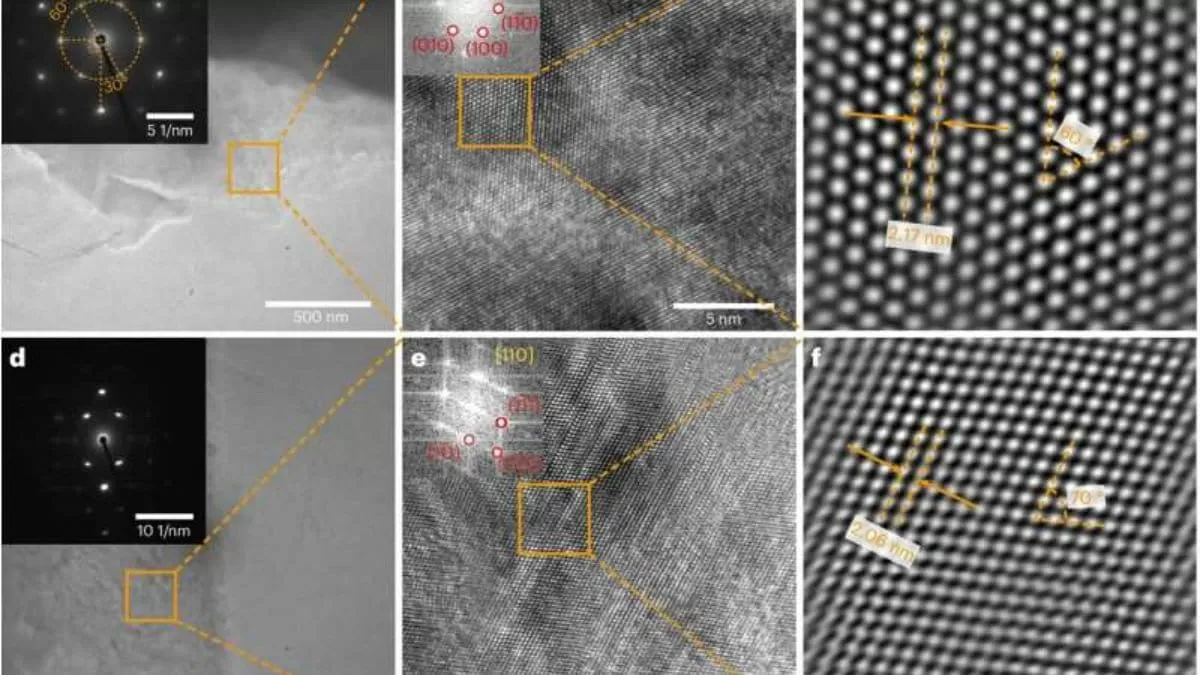
The key to this breakthrough lies in the unique structure of the synthetic diamond. Unlike natural diamonds, which have a cubic lattice structure, this new diamond has a hexagonal lattice structure. This means that the atoms are arranged in a different pattern, making it much stronger and resistant to external forces. The hexagonal lattice also allows for the diamond to be grown in larger sizes, making it more suitable for industrial use.
But what makes this diamond unsuitable for jewellery, you may ask? Well, the answer lies in its color. Natural diamonds get their color from impurities in the crystal, whereas this synthetic diamond has a pure white color due to its perfect structure. This may not be appealing to those who are used to the traditional sparkling and colorful diamonds. However, the potential industrial applications of this diamond far outweigh its unsuitability for jewellery.
One of the most significant advantages of this synthetic diamond is its potential use in cutting and drilling tools. The extreme hardness and durability of this diamond make it ideal for use in industrial processes that require precision and strength. It can easily cut through materials that are too tough for natural diamonds, such as hardened steel and concrete. This could revolutionize the construction and manufacturing industries, making them more efficient and cost-effective.
Another potential application of this diamond is in the electronics industry. With the rise of technology, there is a growing demand for materials that can withstand high temperatures and pressures. This synthetic diamond could be used in electronic devices to dissipate heat and improve their performance. It could also be used in high-power switches and transistors, making them more durable and efficient.
The medical industry could also benefit from this synthetic diamond. Its extreme hardness and durability make it suitable for use in surgical tools, such as scalpels and drills. These tools need to be sharp and strong to perform delicate and precise procedures, and this diamond could provide just that. It could also be used in prosthetics, as it is biocompatible and can withstand the wear and tear of everyday use.
Moreover, this synthetic diamond could also have environmental benefits. As it is made from carbon, it is a more sustainable alternative to natural diamonds, which are mined from the earth. The mining of diamonds has a significant impact on the environment, and this synthetic diamond could reduce that impact by providing a more eco-friendly option.
The potential uses of this synthetic diamond are endless, and its development marks a significant milestone in the field of materials science. It is a testament to the ingenuity and perseverance of scientists who are constantly pushing the boundaries of what is possible. This diamond may not be suitable for jewellery, but its strength and durability make it a game-changer in various industries.
The process of creating this synthetic diamond is also worth mentioning. It involves placing a layer of graphene on top of a substrate and then subjecting it to high pressures and temperatures. This causes the carbon atoms in the graphene to rearrange and form a diamond structure. This process is relatively simple and can be easily scaled up for industrial production.
In conclusion, the creation of a synthetic diamond with a hexagonal lattice structure is a remarkable achievement that has the potential to transform various industries. Its extreme hardness and durability make it suitable for use in cutting and drilling tools, electronics, medical devices, and even as a sustainable alternative to natural diamonds. This breakthrough is a testament to the endless possibilities of science and technology and gives us a glimpse into a future where synthetic diamonds could be a part of our everyday lives.